
After watching the 2018 release of The Cloverfield Paradox (SPOILER ALERT), I was not so puzzled about the paradox itself, but rather why Tam (the Cloverfield station engineer), after being trapped in a chamber flooded with water, had completely frozen following an explosion that exposed her to the empty vacuum of space. It wasn’t as though the station had teleported to the middle of nowhere, they were just on the opposite of the Sun to Earth. You would think the star responsible for the fate of the entire solar system could spare enough energy to keep a chamber of water warm? Let’s see if we can figure out what happened.
Both the atmospheric pressure and temperature of outer space drop exponentially when in comparison to Earth, as the only form of heat transfer that can occur in space is radiation. With nearly no matter to absorb the energy of the Sun (aside from larger celestial bodies), it’s no wonder why space is so cold. When a chamber full of water is suddenly exposed to this environment, it would first be brought to a violent boil and vaporize due to the rapid drop in atmospheric pressure, which then in its gaseous form would immediately freeze into ice crystals.
Now, that was a rather short summary of the entire process. If you’re interested in what goes on behind the scenes just keep on reading!
Why do we call outer space a vacuum?

Image by NASA
A vacuum in short is a region of space that’s void of any matter, which by that definition might describe our conventional understanding of outer space. But in reality, outer space is not a perfect vacuum. Aside from the existence of celestial bodies like the moon, planets, or stars, space is actually sparsely populated with gas, dust, radiation, and cosmic rays, etc.
When we compare such a unique environment to the atmosphere of Earth, which is quite densely populated with a mixture of gases (e.g., oxygen, nitrogen, argon, etc.) that’s packed together by gravity, it would suddenly feel rather lonely.
Why do I say that? Well, if we were to compare the number of molecules in space with those on Earth, we would have lost before the game started. But if we were to compare their density and weight, things might turn out a little different.
There is one thing Earth has that the emptiness of space cannot hope to compare – Gravity. And it is because of this mysterious force that the atmospheric pressure on Earth decreases with increasing altitude. The gravitational attraction exerted on the molecules at sea level would be greater than the attraction exerted on the molecules at the top of Mt. Everest.
In this sense, the closer the molecules are to the Earth’s core, the heavier they become. This causes a decrease in distance between each molecule and increases the pressure as they become more compact. But that’s not all. Despite weighing less at a higher altitude, the molecules on top also exert their own weight for those at the bottom, which leads to further compression and pressurization.
But fear not, there are no such worries in the unending bounds of space. Although that’s not to say gravity doesn’t exist. Gravitational waves are everywhere. Between the moon and our ocean, other stars and their own orbiting planets, supermassive black holes, etc., which altogether would only approximate an average pressure of 1.322 x 10^-11 Pa, effectively making space a near-perfect vacuum.
What happens to water in a near-perfect vacuum?
Don’t worry, I haven’t forgotten the purpose of this article. Like I said, in outer space, before water can freeze into ice crystals, it must boil and vaporize first. But why does it have to occur in this order?
Well, referencing back to the scene in The Cloverfield Paradox, the water was trapped inside a chamber that had being pressurized to simulate living conditions on Earth. But after a hole had opened up exposing the chamber to space, all the water was suddenly shifted into a vacuum like environment.
This sudden drop in pressure could only mean one thing – a lower boiling point. And to understand why we have to first learn about a little special something called vapor pressure.
The vapor pressure of a substance such as water is the pressure caused by the evaporation of liquid water to its gaseous state – water vapor. But this can actually occur without increasing the temperature of the water!
Not all molecules of a liquid have the same amount of energy. At a fixed temperature, some molecules have enough energy to break free from the attraction of their neighbors into another state, while the others are stuck. That’s why when we heat up water, some escape as steam/water vapor while the rest are stuck in the pot (or whatever you use to boil water with).
Although, this concept can be better understood in a closed system (container). Let’s take a half-filled plastic water bottle as an example. If we were to leave it out in the open at room temperature with the lid on for half a day, what would happen?

Right, we probably have all seen this before. There would be vapor clinging to the walls of the plastic bottle. As time passes, we then observe this gas condensing back into droplets of water as the empty areas of the bottle have officially run out of room to hold any more vapor molecules.
As such, within this closed system, the vapor pressure would indicate the amount of pressure exerted by the vapor molecules on the walls of the bottle, as well as the equilibrium rate at which the water molecules are changing back and forth between its liquid and gaseous state.
But what if we were to heat this water up under the sun?
First of all, as more heat and energy are supplied to liquid water, its average kinetic energy increases. Which at the same time also increases the probability of more liquid water molecules having enough kinetic energy (moving faster and faster) to break free from the attraction of their neighbors (thus becoming water vapor). Then, as more and more water molecules break away from their liquid state within this closed bottle system, the pressure exerted by this vapor also increases.
What is boiling? How does water boil in space?
The common misconception is that boiling water has to be hot, but water can actually begin to boil at room temperature. ‘Boiling’ does not indicate the temperature of a fluid, but rather the change of a fluid from its liquid to a gaseous state, as well as the equalization between its vapor pressure and the atmospheric pressure exerted on it – the formation of bubbles.

When boiling occurs, the water molecules closest to the source of heat begin to escape from their friends (spreading out) and change into pockets/bubbles of water vapor. But the only way for this bubble to retain its shape and escape the surface is if its internal vapor pressure is equal to the outside pressure exerted on it, or else it would either burst or collapse on itself.
This external pressure is generally affected by two things – atmospheric pressure and water pressure. Most of the time, water pressure is negligible as it depends solely on the density and depth of water, which for a typical glass of water, the pressure at 2 centimeters below the surface is only a 0.2 % increase over the atmospheric pressure (Wired).
Here is where everything ties together. For water to start boiling, all we really need to do is either increase its temperature until the vapor pressure is equal to the atmospheric pressure or decrease the atmospheric pressure until it is equal to the vapor pressure of water at its current temperature.
So, what happens in outer space? As a near-perfect vacuum, space effectively has zero atmospheric pressure. This means water will boil and vaporize (turn into water vapor) INSTANTLY as there is no external pressure to counteract water’s own vapor pressure.
How is heat transferred in outer space?
There are many processes by which heat loss can occur on Earth, but they are not all applicable when transferred into a near-vacuum environment.
The most basic types of heat transfer include conduction, convection, and radiation.
Conduction
Conduction is the transfer of heat by touch or direct contact, and this energy will always transport from hot to cold through the vibration of molecules.
So, does conduction occur in outer space? Well, the Sun is very, very hot with a surface temperature of approximately 5600 degrees Celsius! But since outer space exists as a near-vacuum, all the available particles are too sparsely populated to conduct the heat from the sun via direct contact.
Convection
The process of convection occurs through the flow of atoms from a hot to cold region, which can happen in both liquids and gases. Although there are no naturally occurring liquids in outer space, gases still exist. But we also have to remember that space is almost a perfect vacuum, so these gaseous regions are very sparsely populated across the universe; definitely not close enough to convect enough heat from the stars to make a difference.
You just might feel a little warmer if you were to ever traverse through a cloud of gases.
Radiation
We know dust particles and gases definitely have their own place in outer space, but how exactly do they gain their energy/heat without the process of conduction or convection?
This only leaves us with one option, radiation. But we are going to look at one specific type of radiation today – thermal radiation. All matter with a temperature greater than absolute zero (the lowest possible temperature) generates heat. And this heat causes the movement/vibrations of atoms, which is converted and emitted as electromagnetic radiation (EMR).
The types of EMR emitted by the sun include visible light, UV light, infrared, radio waves, X-rays, etc., which can all be absorbed for energy. But it is infrared radiation that we humans emit and feel as heat or warmth.
But for the sake of this article, the most important thing to know is water in space also releases its heat/energy via thermal radiation.
How does water freeze?
Freezing occurs when the molecules of a liquid lose so much energy (get so cold) that they start moving slow enough to stay attracted to each other. By remaining in these fixed positions, the molecules form a solid crystal.
For fresh/pure water, freezing occurs at 32 degrees Fahrenheit or 0 degrees Celsius, but the rate at which water cools depends on its surroundings (on Earth).

You might think because the temperature difference between outer space (-270.45 degrees Celsius) and a cup of room temperature water (20 degrees Celsius) is huge and that as a result, the water would freeze instantly if it were present in space.
You know what… you’re absolutely right! Although, maybe not for the reasons you might be thinking.
Newton’s law of cooling states that the rate of heat loss of a body is directly proportional to the difference in temperature between the body and its surroundings, which means the bigger the temperature difference the faster the heat loss! But… this unfortunately only holds true for very small temperature differences during heat transfer by thermal radiation. And as thermal radiation is the only way for heat loss to occur in outer space, the temperature difference doesn’t really mean much.
Another important physical property of water we have to understand is its specific heat capacity, which is actually one of the highest out of all known substances! Specific heat capacity can be defined by the amount of energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius (USGS). This means that water has to absorb a large amount of heat/energy to not only raise its temperature but also decrease it.
With all this information, let’s see if we can put everything together.
Why does water boil before freezing in space? Why does it freeze?
The general logic is as follows: Dropping the pressure surrounding a substance of a fixed temperature would cause it to rapidly boil (refer back to the section ‘what happens to water in a near-perfect vacuum’). And dropping the temperature of a substance at a fixed pressure would cause it to freeze (as can be seen regularly on Earth).
But, due to the lack of heat transfer processes in outer space, objects in space tend to stay at the same temperature for a very long time, which paired together with the high specific heat capacity of liquid water, would actually take a while before it could lose enough energy to freeze at near-zero pressure.
That is unless there was a way to expose each water molecule to the sub-zero environment of space.
Oh, wait… there is, and it has already happened! As the water boils and vaporizes, the molecules of the water vapor separate and spreads much further apart. This increases the surface area each water molecule has to exchange heat with its surroundings while decreasing the amount of energy required for the molecules to reach freezing temperatures.
But why less energy you might be thinking? Well, like mentioned above, specific heat capacity is the amount of heat in Joules needed to raise the temperature of 1 gram of a substance by 1 degree Celsius. But this specific heat is different for water in its liquid vs gaseous state. Within a water vapor molecule, there are less bonds that needs breaking as they have already been broken during the liquid water’s change in state, so less energy is required to further heat up these molecules (and break more bonds). Which vice versa can be understood as less energy is required to cool the vapor molecules down, effectively decreasing the time needed to reach freezing temperatures.
In the end, this means that when liquid water of a fixed temperature arrives in outer space, it would boil and vaporize rapidly due to the drop in pressure, giving away into a fine mist of water vapor which freezes instantly into ice crystals due to an increase in surface area and decrease in specific heat capacity.
This can also be seen in the phase diagram for water which can provide more specific numbers:
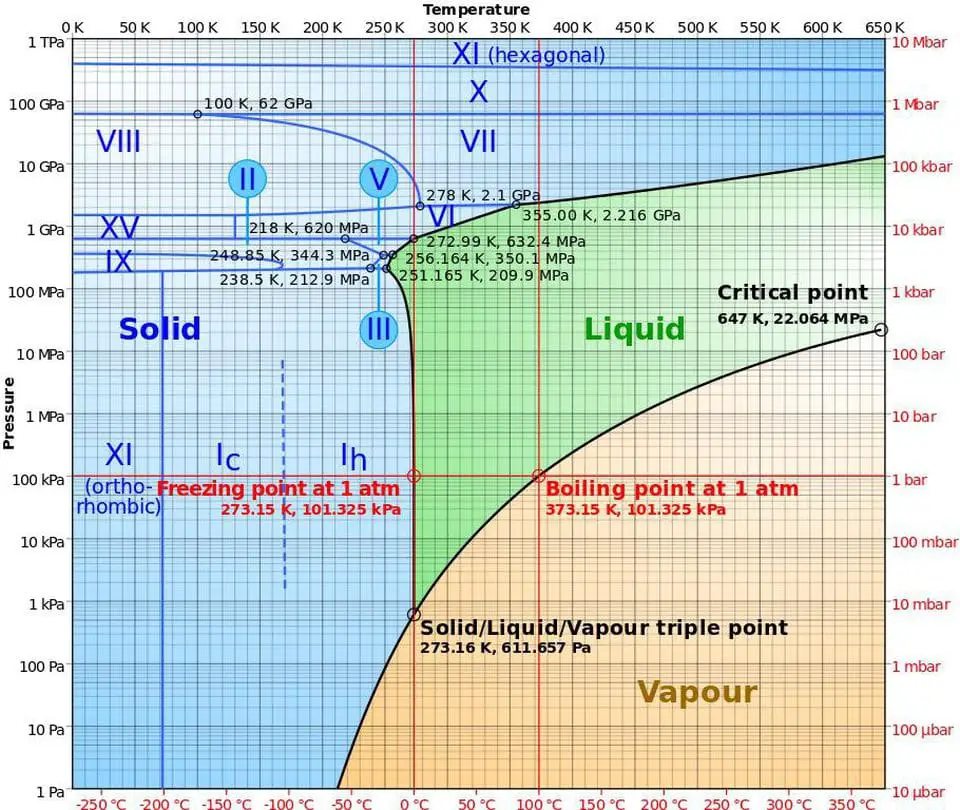
It might look overwhelming, but it is really not. We only have to focus on one single point in this graph.
Since outer space contains a near-vacuum environment, we start this process by looking at the bottom left corner of the graph, where the pressure is the lowest (1 Pascal). Then, to find the temperature required for water vapor to skip its liquid phase right into its solid state (ice), we have to find the intersection between the vapor and solid region at 1 Pascal, which brings us to a temperature of approximately 210 Kelvins or –63.15 degree Celsius.
Compared to the temperature of outer space (-270.45 degree Celsius), which is leagues colder than the temperature needed for the phase change to occur: the collection of water vapor will cool and freeze almost instantly.
I hope this article helped for everyone that was just as curious as I am!
Who knew water was so complicated.
